Unlock the editor’s digest in free
FT editor, Rolla Khalif, selects his favorite stories in this weekly newsletter.
China’s obesity crisis is a significant profit. More than half of the Chinese adults are overweight or obese. This is a personality that will be expected to kill two -thirds by 2030. It is about 900 900Mn people, a huge market for the United States, and is running to dominate pharmaceutical groups.
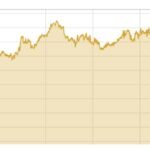
This is a promotion for Novo Nordsk. Danish drug makers are already enjoying the global air by treating their blockbuster weight loss. Last year, revenue from the country increased by 13 % to DKR 18.5bn ($ 2.6bn), though Vigovo was started in November 2024 alone.
But this golden age can be short. The Navo Nordsesk market dominance in China comes with the expiry date: March 20, 2026. When the patent currently protects semigloods – the active component of weight loss drugs like Ozampic and Vigo – expires, and doors open for flooding local competitors. And they are more than ready.
At least 15 Chinese drug makers are racing to sell their own versions of semaglotide. Of these, Hurdong is leading the Medicine Charge, which is already in the final of the clinical trials. Pir Hangzhou Jiwan Gene Engineering has applied for approval of a treatment that claims that similar medical utility and protection is protected. A long list of both biotech startups and the drug maker stands behind them with their replacement.
The opportunity is not limited to China. In countries like India, the increasing demand for weight loss pharmaceuticals, where obesity is a growing concern for public health, means that there is room for development out of the house. During the last six months, shares of Huadong Medicine have increased by one quarter, while Hangzhou Jiwan is at least third of last year, reflecting the expectations of their weight loss drugs.
Novo Nordsk’s patent loss is not just a business challenge – this is part of a major change in China’s pharma sector. For decades, local drug makers were primarily known as generic producers, who, instead of themselves, produced low -cost copies of existing medicines, rather than innovation. But in recent years, local drug makers have been advancing China, in which groups such as Bejin and Jinni Biovasins have made progress in areas such as ontology and immunotherapy, which secure US and European regulatory approval.
If local drug makers have shown that they can successfully scale and commercials Homgon semaglotide alternatives, it will represent another step in continuous transition from China’s low -cost generous manufacturer in a more competitive presence in the global pharma industry.
jun.yoon@ft.com